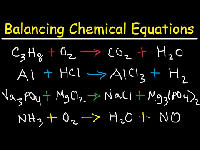
Chemistry
Welcome to Chemistry
Chemistry is a branch of Physical Science. It focuses on matter, and students will learn about the composition and identification of matter. Study of properties, interactions, combination, and changes of matter will also be included in the course.
Chemistry is broken up into the following 8 units:
The following Pennsylvania State Standards will be taught in the course:
Atomic Theory
The theory of the atom, atomic models, and the scientists who contributed to modern atomic theory
- CHEM.A.2.1.1. - Atomic Theory And Models - Describe the evolution of atomic theory leading to the current model of the atom based on the works of Dalton, Thomson, Rutherford, and Bohr.
- CHEM.A.2.1.2. - Isotopes And Mass Number - Differentiate between the mass number of an isotope and the average atomic mass of an element.
- CHEM.A.2.2.1. - Electron Configuration - Predict the ground state electronic configuration and/or orbital diagram for a given atom or ion.
- CHEM.A.2.2.2. - Characteristics Of Atoms And Ions - Predict characteristics of an atom or an ion based on its location on the periodic table (e.g., number of valence electrons, potential types of bonds, reactivity).
Electrons and Periodicity
All about electrons, the periodic table, and periodic trends
- CHEM.A.2.2.3. - Relationship: Electron Configuration And Atomic Structure - Explain the relationship between the electron configuration and the atomic structure of a given atom or ion (e.g., energy levels and/or orbitals with electrons, distribution of electrons in orbitals, shapes of orbitals).
- CHEM.A.2.2.4. - Emission Spectra - Relate the existence of quantized energy levels to atomic emission spectra.
- CHEM.A.2.3.2. - Periodic Properties Of Elements -Compare and/or predict the properties (e.g., electron affinity, ionization energy, chemical reactivity, electronegativity, atomic radius) of selected elements by using their locations on the periodic table and known trends.
- CHEM.A.2.3.2. - Periodic Properties Of Elements -Compare and/or predict the properties (e.g., electron affinity, ionization energy, chemical reactivity, electronegativity, atomic radius) of selected elements by using their locations on the periodic table and known trends.
Solutions
Properties of solutions, differences between them, and how they can be affected
- CHEM.A.1.2.1. - Properties Of Solutions - Compare properties of solutions containing ionic or molecular solutes (e.g., dissolving, dissociating).
- CHEM.A.1.2.2. - Homogeneous And Heterogeneous Mixtures - Differentiate between homogeneous and heterogeneous mixtures (e.g., how such mixtures can be separated).
- CHEM.A.1.2.3. - Affecting Solubility - Describe how factors (e.g., temperature, concentration, surface area) can affect solubility.
- CHEM.A.1.2.4. - Solution Concentration - Describe various ways that concentration can be expressed and calculated (e.g., molarity, percent by mass, percent by volume).
- CHEM.A.1.2.5. - Chemical Bonds And Solubility - Describe how chemical bonding can affect whether a substance dissolves in a given liquid.
Properties and Nomenclature
Chemical and physical properties and changes, and how to name compounds
- CHEM.A.1.1.1. - Physical And Chemical Changes - Classify physical or chemical changes within a system in terms of matter and/or energy.
- CHEM.A.1.1.2. - Classify Observations - Classify observations as qualitative and/or quantitative.
- CHEM.A.1.1.3. - Significant Figures - Utilize significant figures to communicate the uncertainty in a quantitative observation.
- CHEM.A.1.1.4. - Physical Properties And Structure - Relate the physical properties of matter to its atomic or molecular structure.
- CHEM.A.1.1.5. - Naming And Writing Formulas - Apply a systematic set of rules (IUPAC) for naming compounds and writing chemical formulas (e.g., binary covalent, binary ionic, ionic compounds containing polyatomic ions).
The Mole
Special unit of measurement, formulas, proportions, and percent composition
- CHEM.B.1.1.1. - The Mole Concept - Apply the mole concept to representative particles (e.g., counting, determining mass of atoms, ions, molecules, and/or formula units).
- CHEM.B.1.2.1. - Empirical And Molecular Formulas - Determine the empirical and molecular formulas of compounds.
- CHEM.B.1.2.2. - Law Of Definite Proportions - Apply the law of definite proportions to the classification of elements and compounds as pure substances.
- CHEM.B.1.2.3. - Percent Composition - Relate the percent composition and mass of each element present in a compound.
Bonding
How atoms come together to form new substances
- CHEM.B.1.3.1. - Ionic And Covalent Bonding - Explain how atoms combine to form compounds through ionic and covalent bonding.
- CHEM.B.1.3.2. - Classify Chemical Bonds - Classify a bond as being polar covalent, non‐polar covalent, or ionic.
- CHEM.B.1.3.3. - Predict Polarity - Use illustrations to predict the polarity of a molecule.
- CHEM.B.1.4.1. - Modeling Compounds And Chemical Formulas - Recognize and describe different types of models that can be used to illustrate the bonds that hold atoms together in a compound (e.g., computer models, ball‐and‐stick models, graphical models, solid‐sphere models, structural formulas, skeletal formulas, Lewis dot structures).
- CHEM.B.1.4.2. - Lewis Dot Structures - Utilize Lewis dot structures to predict the structure and bonding in simple compounds.
Chemical Reactions
Reaction types, predicting products, limiting and excess reactants
- CHEM.B.2.1.1. - Limiting And Excess Reactants - Describe the roles of limiting and excess reactants in chemical reactions.
- CHEM.B.2.1.2. - Calculate Amounts: Reactants And Products - Use stoichiometric relationships to calculate the amounts of reactants and products involved in a chemical reaction.
- CHEM.B.2.1.3. - Classify Chemical Reactions - Classify reactions as synthesis, decomposition, single replacement, double replacement, or combustion.
- CHEM.B.2.1.4. - Predict Products Of Chemical Reactions - Predict products of simple chemical reactions (e.g., synthesis, decomposition, single replacement, double replacement, combustion).
Balancing Chemical Equations and Gas Laws
Balancing both sides of a chemical equation, and all about how gases work
- CHEM.B.2.1.5.) Balance Equations - Balance chemical equations by applying the Law of Conservation of Matter.
- CHEM.B.2.2.1. - Gas Laws - Utilize mathematical relationships to predict changes in the number of particles, the temperature, the pressure, and the volume in a gaseous system (i.e., Boyle's law, Charles's law, Dalton's law of partial pressures, the combined gas law, and the ideal gas law).
- CHEM.B.2.2.2. - Gases: Molar Volume - Predict the amounts of reactants and products involved in a chemical reaction using molar volume of a gas at STP.